
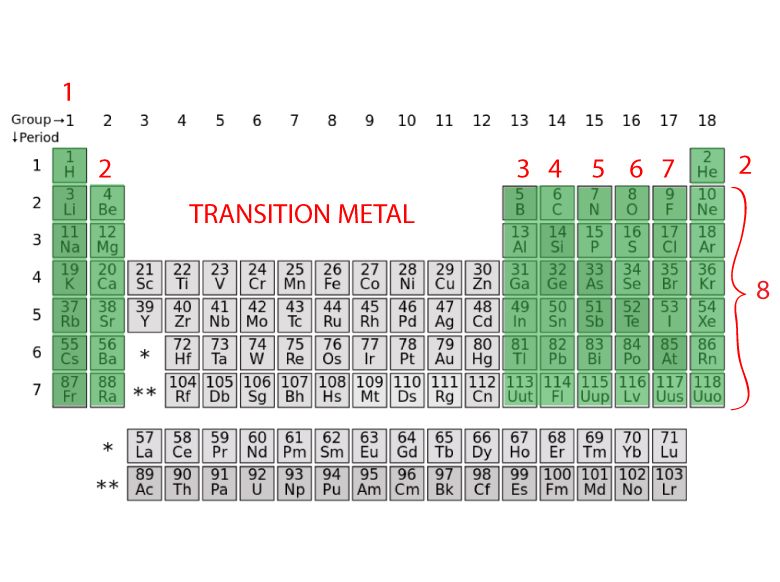
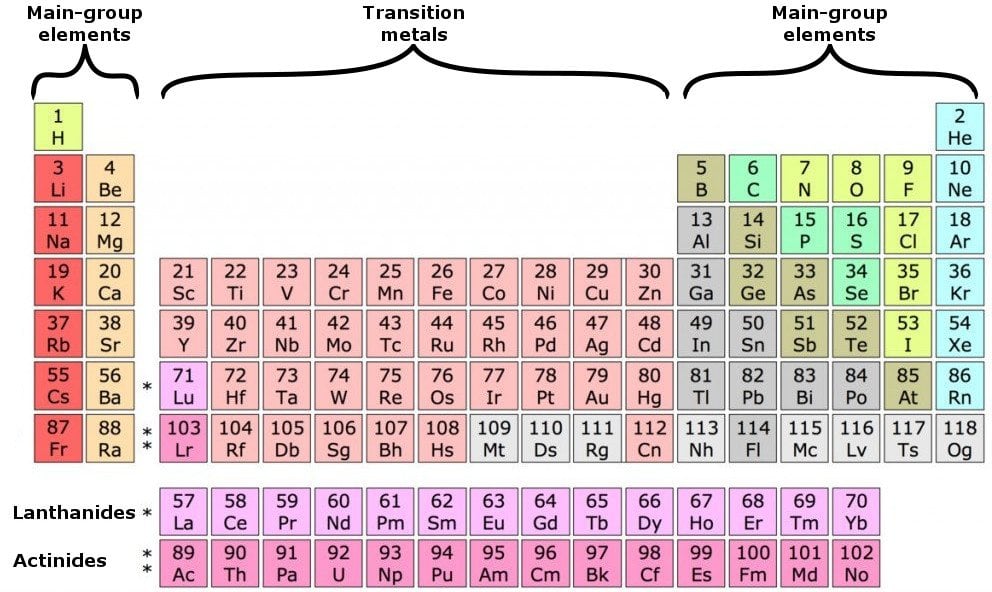
The rule is inapplicable to the transition and inner transition elements (we’ll get to that reason in a minute). However, this is only true for the main group elements-the elements inhabiting groups 1-2 and 13-18. Specifically, the number in the ones’ place. While the period number indicates the number of shells, the group number indicates the number of valence electrons in the outermost shell. So, what is the significance of the group number? Valence electrons of elements other than transition elements – the main group elements The period number (row number, to remind you) in which an element can be found indicates the number of shells encircling its nucleus. While valence electrons across a period incrementally climb by one, the number of shells remain the same. When we go down a group, the number of valence electrons remains the same, although the number of shells increases. The transition elements form a bridge, or perpetuate the transition between the elements in Groups 2 and 13. There are 7 rows in the subtable above and 2 rows distinguishing the rarer elements below. The table contains 18 columns in total, formally known as groups, as well as rows, formally known as periods. The latter two are also referred to as inner transition elements. The elements are divided into four categories: main group elements, transition elements, lanthanides and actinides. The elements are arranged from left to right in ascending order of their atomic numbers or the number of protons or electrons they contain. The periodic table is a clean arrangement of all elements discovered so far. In order to determine the number of valence electrons of an element, we only have to refer to the periodic table and search for the position of the element in it. This is a more generalized approach that only requires summoning one small resplendent rectangular sheet of paper - the periodic table. However, there’s no need to fret, as there’s a much simpler manner of determining this coveted number.
However, this would be an extremely laborious task, as we may have to dig through textbooks to find configurations we do not know. The most basic method would be to refer to the atomic configuration of an element and simply count the electrons in the outermost shell. Irrespective of the type of chemical bond between atoms, be it an ionic, covalent or metallic bond, changes in the atomic structure are limited to the electrons in the outermost shell, i.e. In other words, these are the electrons that can be gained or lost during a chemical reaction. Valence electrons are the electrons that are located in the outermost shell of an atom.


 0 kommentar(er)
0 kommentar(er)
